Is Hno3 Acid Or Base
 |
|
Acid-base reactions are ubiquitous. In aqueous solutions acids increase the hydrogen ion (H+) concentration. On the other hand bases increment the hydroxide ion (OH-) concentration. When an acrid and a base react in an aqueous solution the H+ and OH- ions combine to form h2o. These ions thus "neutralize" one another:
Nearly acids accept the general formula HA, where A- is an anion and most bases take the form BOH, where B+ is an advisable cation. Acids and bases tin be grouped into two general types: strong and weak acids and bases. The departure between the two is straightforward: a stiff acrid in a water solution decomposes 100% into a proton (H+) and anion (A-)
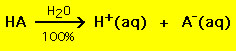
On the other hand most weak acids decompose significantly less than 100% in a water solution:
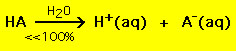 .
.In other words well-nigh weak acid molecules stay intact in h2o. Similar chemic equations agree for stiff and weak bases.
There are only a few weak acids and bases, they are:
| | |
| HCl (hydrochloric acid) HNO3 (nitric acid) HClO4 (perchloric acid) H2SO4 (sulfuric acid) | NaOH (sodium hydroxide) KOH (potassium hydroxide) Ca(OH)2 (calcium hydroxide) |
All other acids and bases are weak. A weak acrid results from any anion. Examples are given below
| | |
| F- (fluoride) Br- (bromide) I- (iodide) HCO3- (bicarbonate) C2H3O2- (acetate) MnO4- (permanganate) PO4-three (phosphate) CrO4-two (chromate) CN- (cyanide) NO2- (nitrite) SO3-2 (sulfite) | HF (hydrofluoric acid) HBr (hydrobromic acid) Hi (hydroiodic acid) H2CO3 (carbonic acid) HC2H3O2 (acerb acid) HMnO4 (permanganic acid) H3PO4 (phosphoric acrid) H2CrO4 (chromic acid) HCN (hydrocyanic acid) HNO2 (nitrous acid) H2SO3 (sulfurous acid) |
In a typical acid/base reaction the acid and base of operations react to form a salt and water east.m., hydrocyanic acid and sodium hydroxide:
Is Hno3 Acid Or Base,
Source: https://cpanhd.sitehost.iu.edu/C101webnotes/chemical%20reactions/acidbase.html
Posted by: moralesknoid1942.blogspot.com


0 Response to "Is Hno3 Acid Or Base"
Post a Comment